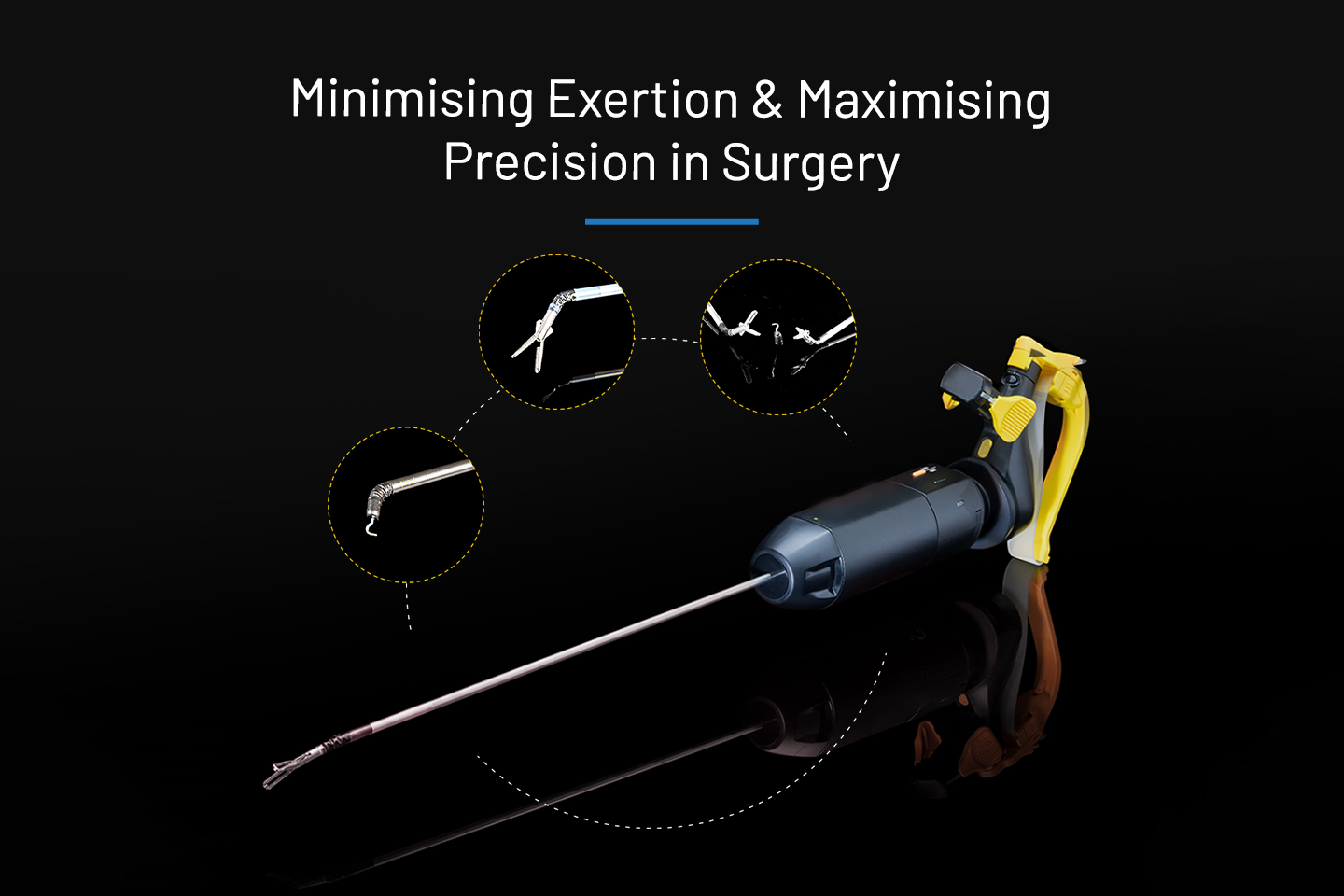
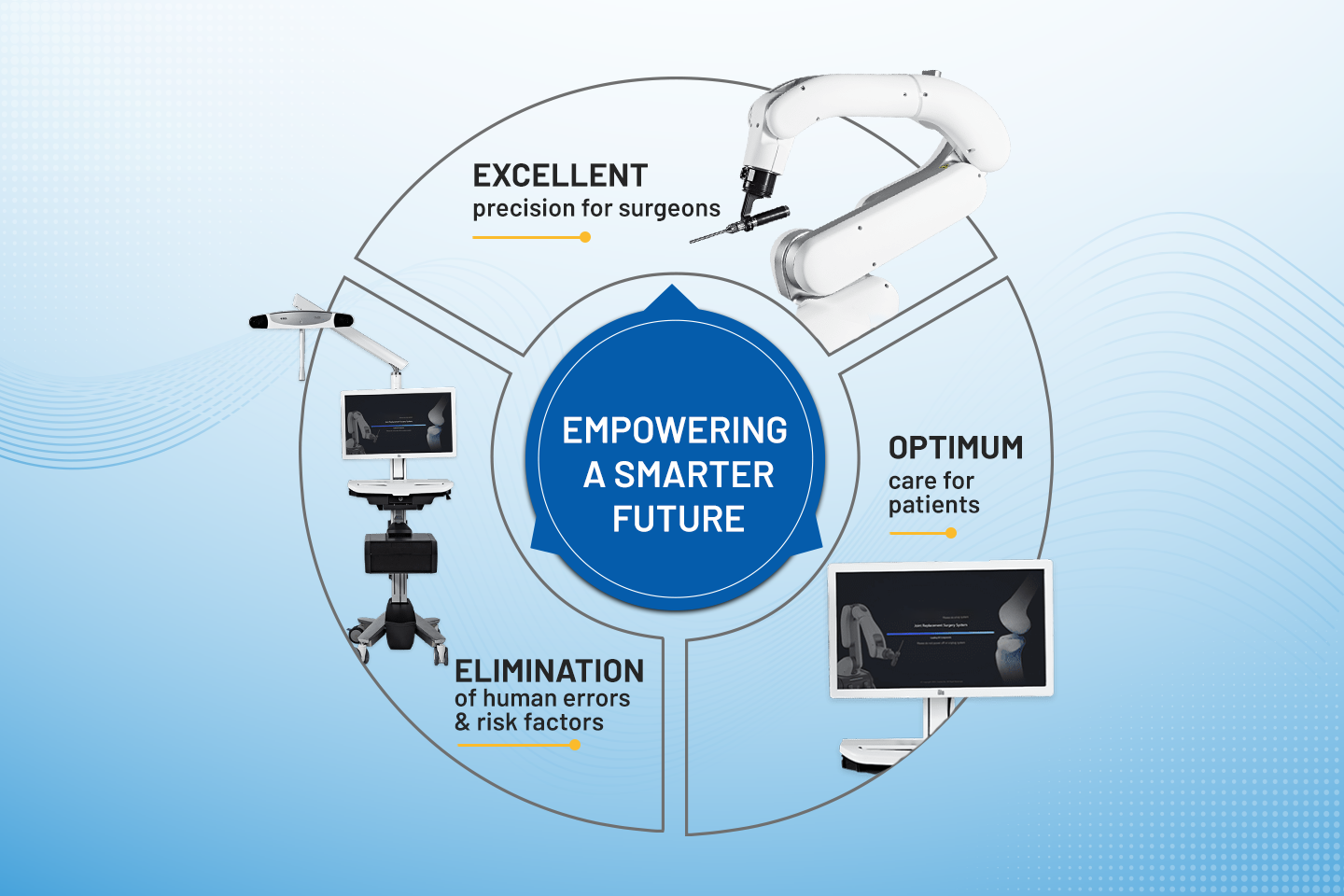
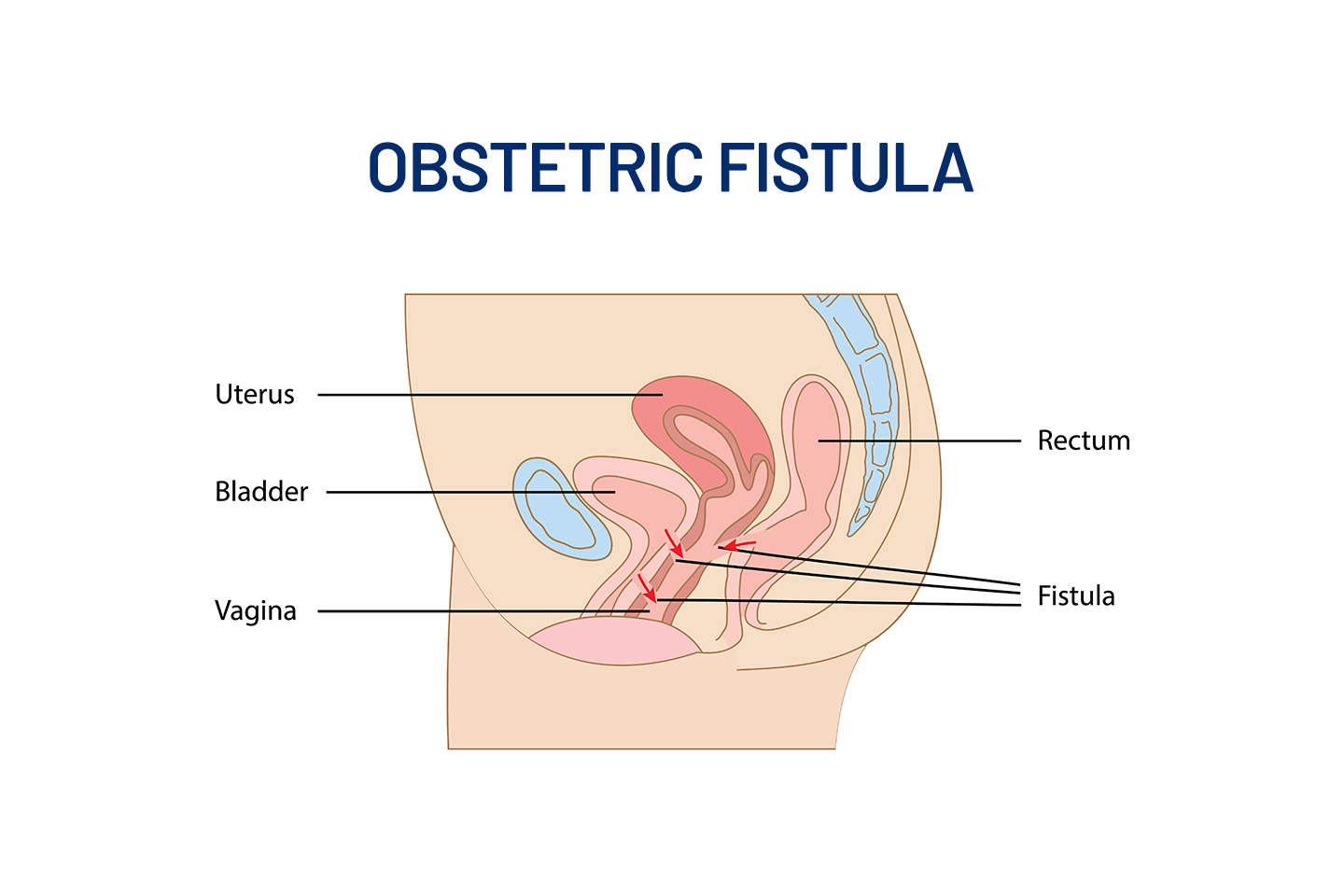
Medical Devices
Meril Life Sciences: Leading the path to a growing Indian Medtech sector

The Indian medical device sector is the fourth largest medical device sector in Asia, currently valued at 5.2 billion dollars and growing by 16% every year. Such a market provides manufacturers and investors with a wide range of opportunities. This growth is not just due to the size of the market but also because of regulatory makeovers such as the Indian Medical Devices Rules, passed in 2017. Being one of the industry leaders in the Medtech sector, Meril Life Sciences has personally felt the changes in the industry first-hand. Let us take a closer look at the changing face of Indian Medtech and the role of Meril Life Sciences in this change.
The changing face of Indian Medtech
The Indian Medical Devices Rules, 2017 seeks to bring the sector and regulations more in-line with global standards and practices. In an official statement, vice president of Meril Life Sciences, Mr. Sanjeev Bhatt said that before 2005 the Indian Medtech space was not granulated. In the last 10 years, the government of India has made a conscious decision to grow the reach of healthcare and hospitals within towns and rural areas. Estimates say, by the year 2025 Indian care providers will need to supply as many as 1.75 million additional beds. This demographic time bomb has prompted many healthcare providers to step into the Medtech and healthcare sectors to fulfill this growing need.
Regulatory changes
According to new regulations, if a medical device has already been approved by countries in the European Union, Australia, Canada, Japan or the US, a clinical investigation is not required for it to be sold in India. Along with this, the Indian government now actively encourages medical visas that permit staying in the country to receive medical care for up to six months. The changing of regulations has also raised the quality and infrastructure levels of Indian healthcare facilities to match international standards.
The role of Meril Life Sciences
In these changing times, Meril Life Sciences recently became the first Indian company to receive European certification for its revolutionary stent technology. In 2019, they presented the results of 14 medical studies at the EuroPCR conference in Paris. Along with exhibiting the company’s recently CE approved devices, The MeRes100 bioresorbable scaffold (BRS) and the Myval transcatheter heart valve(TVH). With 20% of the market share of total knee replacement surgery in India, Meril Life Sciences is at the forefront of the changing face of Indian Medtech.
In India and on a global scale the rise in technology is creating rapid advancements in the healthcare sector. Medtech is at the core of these advancements, revolutionising the way patients are treated and diagnosed. As a global Medtech company, Meril Life Sciences believes that through our ultra-modern manufacturing facilities and the latest cutting-edge medical technology, we have the ability to keep pace with the changing times and make bigger strides in the field of medicine and technology.