OBTURA
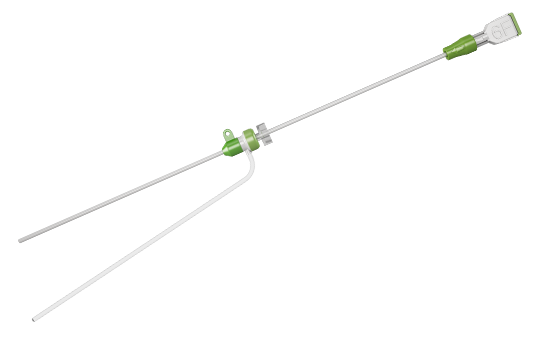
ObturaTM Vascular Closure Device is an effective mechanical system for femoral artery puncture site closure.
Incorporated with a potent sealing system, it provides an assailable sealing with the collagen plug and efficiently designed anchor. ObturaTM Vascular Closure Device is a simple 3-Step closure device making it easy to use and yielding rapid hemostasis.
The ObturaTM VCD closes and sandwiches the arteriotomy between the anchor and collagen sponge. Hemostasis is achieved primarily by the mechanical means of the anchor - arteriotomy - collagen sandwich, which is supplemented by the coagulation inducing properties of the collagen.
The ObturaTM VCD can be used with the existing 6 and 8 French introducer sheath used during the interventional procedures with a working length of up to 12cm.
Benefits
No exchange of introducer sheath : Specially designed ObturaTM VCD is compatible with the standard existing introducer sheath used during the interventional procedures leading to reduced blood loss.
Less procedural time : With only 3 simple procedural steps ObturaTM saves time of the medical practitioner compared to manual compression.
Rapid Hemostasis : Efficient anchor secures with the puncture site leaving no space for blood loss and the collagen plug gives a foolproof hemostasis
90 days absorption : ObturaTM facilitates patient friendly sealing with the implant deployed at the puncture site which is completely absorbed within 90 days.
Easy to use: Ergonomically designed ObturaTM is easy to use with minimal assistance and higher efficiency .
Indication
The ObturaTM Vascular Closure Device is indicated for femoral artery puncture site closure, reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional catheterization procedures using a standard 6F or 8F vascular sheath introducer with up to 12 cm working length.
Product Specifications
| Device Size | 6F and 8F |
| Device Total Length | 205 ±10 mm |
| Device Effective Length | 155 ±10mm |
| Complete Degradation Period | 90 Days |
| Introducer Sheath Compatibility | 6F device compatible with 6F & 7F Introducer Sheath |
| 8F device compatible with 8F & 9F Introducer Sheath |
Size Chart
Ordering Information:
| Product Information | Product Code |
| Obtura Vascular Closure Device 6F | OBT6F |
| Obtura Vascular Closure Device 8F | OBT8F |
Clinical Data
| Study Name | Type of Study | Total Patients | Status | LINKS |
|---|---|---|---|---|
| Obtura VCD-1 Study | Randomized controlled trial | 218 | Ongoing | Know more |
Product IFU
Note: IFU will be displayed after MDR Certification