-
- MERIT TRIALS: HAVE PROVEN THE SAFETY AND EFFICACY OF BIOMIME DES IN MORE THAN 1,700 PATIENTS UPTO 5 YEARS.
- BIOMIME DES HAS PROVEN NON INFERIORITY TO XIENCE DES IN MERIT-V: PUBLISHED IN EUROINTERVENTION (SEP'18) BIOMIME BRANCH USES THE SAME PROVEN PLATFORM.
BIOMIME BRANCH
- Proven benefits of BioMime, now available a dedicated bifurcation stent system. An intuitive design for treating bifurcations lesions.
- Treating Bifurcation lesions with multiple stents can be a long and complex procedure.
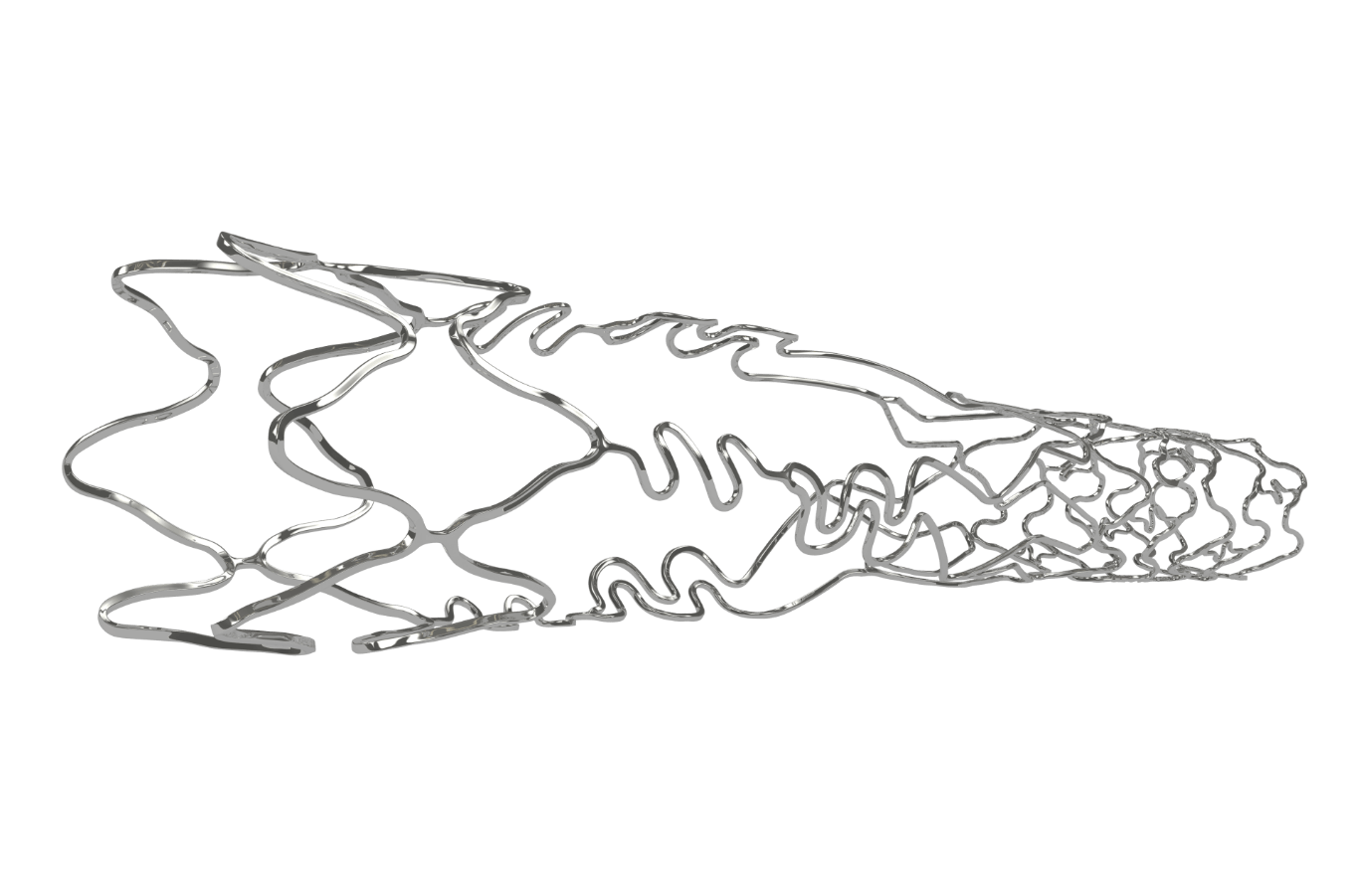
- Biomime BranchTM is a Sirolimus Eluting Coronary Stent System with an ultra-thin 65 µm strut thickness. This next generation SES has a Novel Hybrid Design with a Proximal Main Branch anchoring segment and a Distal tapered Side Branch segment. The two segments are joined through an advanced “Flexi Connector Technology” for continuous access and protection of side branch. The design offers ease of implantation and complete main vessel stent integration.
- Due to the Step-Up Balloon System: The main branch and side branch segments get deployed at one go which reduces the overall procedural time and complexity of multiple hardware usage.
- Post Biomime Branch deployment, the main branch segment can be stented as a regular stent procedure.
Benefits
Product Specifications
| Stent material | Cobalt Chromium L605, Strut Thickness 65 µm |
| Stent architecture | Proximal Main Branch anchoring segment with a Distal tapered Side Branch segment joined through an advanced “Flexi Connector Technology” |
| Drug | Sirolimus |
| Polymer | Biodegradable + Biocompatible |
| Delivery system | Rapid Exchange |
| Drug Dose | 1.25μg/mm2 |
Size Chart
| Diameter / Length | 16 mm | 19 mm | 24 mm | 29 mm |
|---|---|---|---|---|
| Main branch-Side branch | ||||
| 2.50-2.50mm | BBR25025016 | BBR25025019 | BBR25025024 | BBR25025029 |
| 3.00-2.50mm | BBR30025016 | BBR30025019 | BBR30025024 | BBR30025029 |
| 3.50-2.50mm | BBR35025016 | BBR35025019 | BBR35025024 | BBR35025029 |
| 3.50-3.00mm | BBR35030016 | BBR35030019 | BBR35030024 | BBR35030029 |
| 4.00-3.50mm | BBR40035016 | BBR40035019 | BBR40035024 | BBR40035029 |
Clinical Data
| STUDY NAME | TYPE OF STUDY | TOTAL PATIENTS | STATUS | LINKS |
|---|---|---|---|---|
| SPONSOR INITIATED STUDY | ||||
| BioMime Branch-1 Study | Randomized controlled trial | 183 | Ongoing | Know more |
Product IFU
Note: IFU will be displayed after MDR Certification