Meril Presents Positive One-Year Results of the LANDMARK RCT at EuroPCR 2025
Introduction
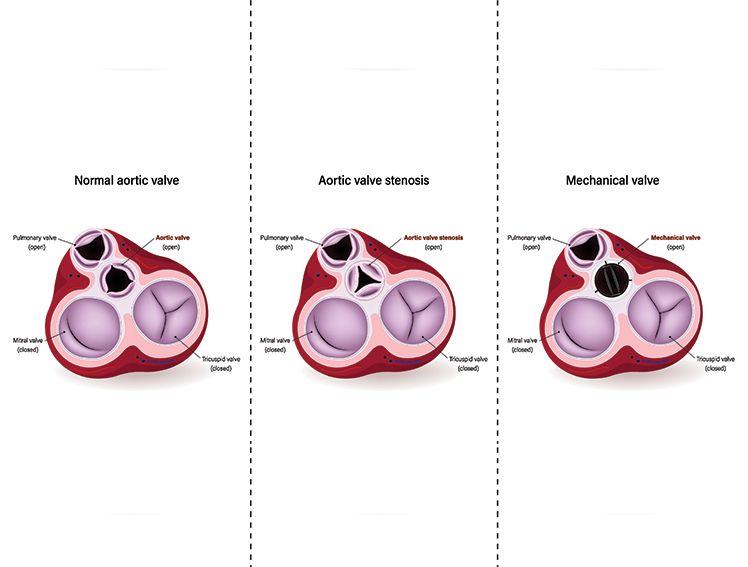
Meril Life Sciences proudly presented the one-year outcomes from the LANDMARK Randomized Controlled Trial (RCT) during the Late-Breaking Trials session at EuroPCR 2025, held in Paris. These findings mark a pivotal step forward in the global evaluation of transcatheter aortic valve implantation (TAVI) technologies and reflect Meril’s unwavering commitment to evidence-based cardiovascular innovation.
Key findings from the 1 year outcomes of the LANDMARK study
The LANDMARK trial is the first global randomized non-inferiority trial comparing Meril’s balloon-expandable Myval Transcatheter Heart Valve (THV) series with two leading commercially available THV platforms:
- The balloon-expandable Sapien THV series, and
- The self-expandable Evolut THV series.
The prospective, open-label, multicenter study enrolled 768 patients across 31 centers in 16 countries, spanning regions such as Europe, Brazil, and New Zealand. The trial design aligns with VARC-3 endpoint definitions, and importantly, it incorporates Quality of Life (QoL) metrics as part of its clinical evaluation.
Key One-Year Findings
- Primary Endpoint – Clinical Efficacy:
Myval demonstrated non-inferiority to contemporary THVs, with a one-year clinical efficacy rate of 13% versus 13.1% for the comparator group (P-value < 0.0001). - Secondary Endpoint – Efficacy + QoL Composite:
Comparable results were recorded:
Myval: 19.5% vs Contemporary THVs: 22.7% (P-value = 0.33). - Survival Rates:
Nearly identical at one year:
Myval: 92.8%, Comparator: 92.9%. - Hemodynamic Stability:
All valve platforms exhibited similar performance in key parameters, including effective orifice area (EOA), mean pressure gradient, and rates of moderate or greater aortic regurgitation. - Quality of Life:
Patients across all arms of the study reported comparable improvements in QoL measures at one year, supporting Myval’s consistent clinical benefit.
Expert Perspectives
Professor Patrick Serruys, Chairman and Study Director, noted:
" This trial reflects a new era in comparative valve research. The meticulous design and adherence to VARC-3 standards, including QoL endpoints, mark it as a pivotal study. The results of the LANDMARK trial represent a meaningful advancement for the global structural Heart community—and most importantly, for patients receiving TAVI. The data not only validates the safety and efficacy of the Myval THV series but also spotlights its adaptability to complex anatomies. This versatility is exactly what clinicians need to deliver precision care across a broad spectrum of patients."
Professor Andreas Baumbach, Global Principal Investigator, added:
" The LANDMARK trial represents a significant step forward in TAVI research. For the first time, we’ve benchmarked Myval against both balloon-expandable and self-expanding platforms in a rigorous randomized setting. The one-year results demonstrate that the new generation Myval THV series can match global standards in safety and efficacy. "
Meril’s Commitment to Global Cardiac Innovation
Speaking on the occasion, Mr. Sanjeev Bhatt, Senior Vice President – Corporate Strategy at Meril, stated:
"The LANDMARK trial represents a significant milestone not just for Meril, but for the global TAVI community. The strong one-year results affirm the Myval THV series as a next-generation solution that delivers consistent safety, clinical efficacy, and improved quality of life across geographies. As the only head-to-head trial of its kind to include both balloon-expandable and self-expanding valves, it reinforces Myval THV series’s versatility and real-world relevance for diverse patient anatomies and healthcare systems. At Meril, we are proud to contribute innovative, evidence-based technologies that are reshaping patient care and expanding access to advanced structural heart therapies worldwide."
The trial will continue to follow patients over a 10-year period, offering valuable insights into long-term valve durability and real-world performance.