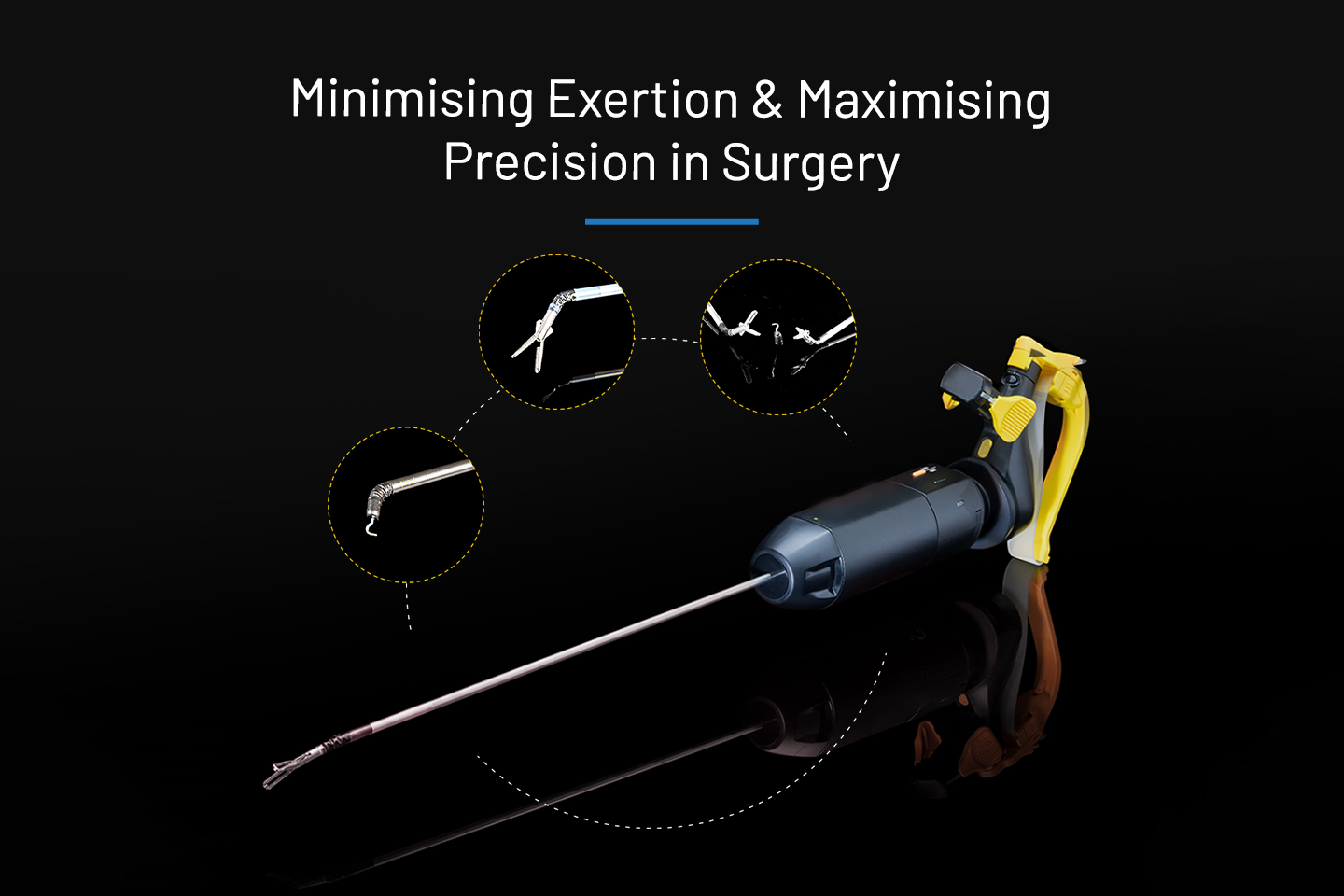
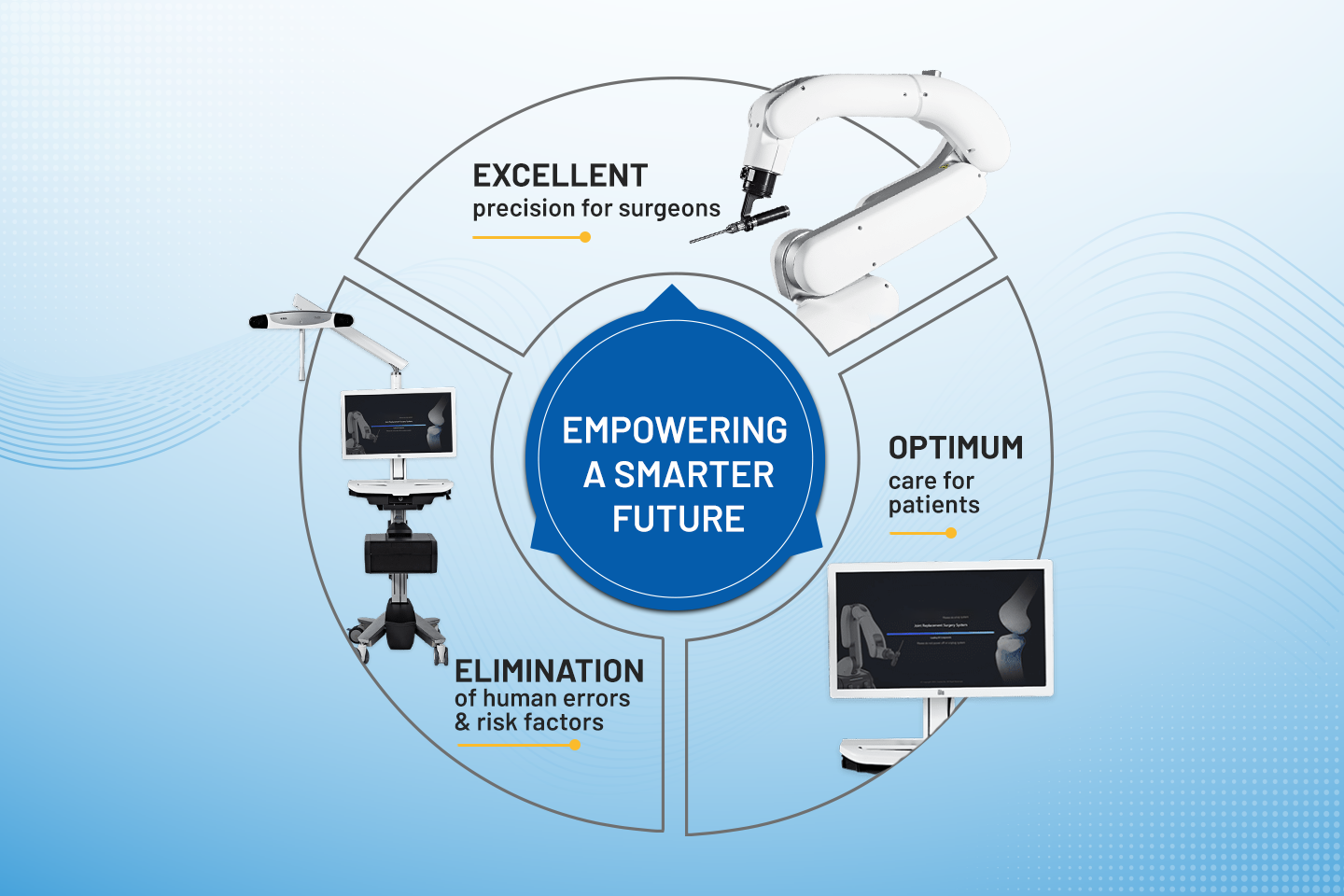
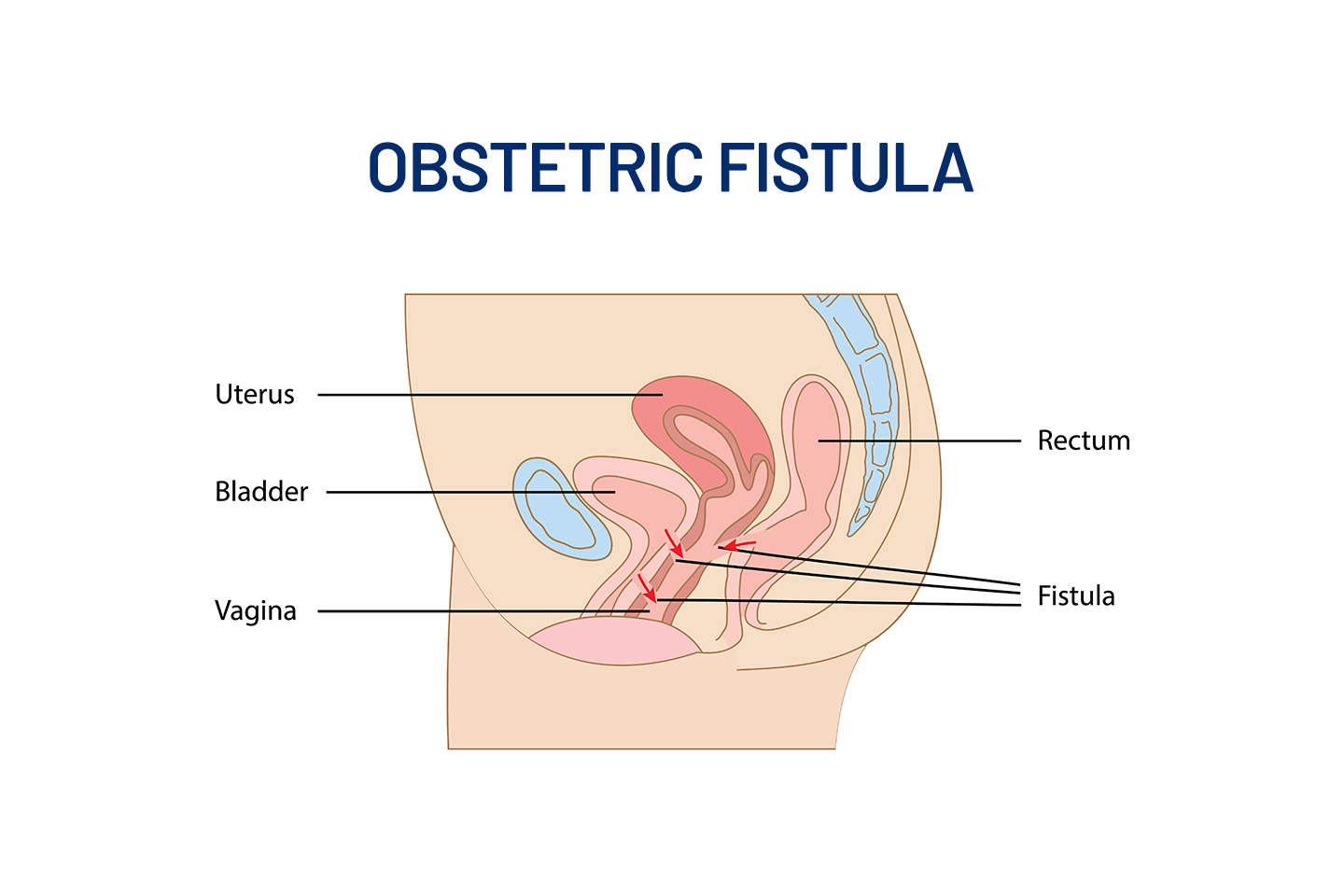
In The News
Meril ‘Adding more to life’ with its innovative initiatives
February 10, 2024

Explore Meril's impactful journey of innovation in healthcare. Learn how our pioneering approach is reshaping the future of medical solutions and adding more to lives.
In The News
Meril Group to invest over Rs.910 Cr for capacity expansion in Gujarat
January 30, 2024

Experience the evolution of healthcare in Gujarat through Meril Group's strategic Rs. 910 Cr investment. Unveil the visionary initiatives reshaping medical advancements and job prospects.
In The News
Meril Life Sciences inks pact with Japan Lifeline to expand presence globally
December 13, 2023

Meril Life Sciences and Japan Lifeline unite for global expansion, with Japan Lifeline securing exclusive rights to introduce Meril's transcatheter heart valve, Myval Octacor, following PMDA approval in Japan.