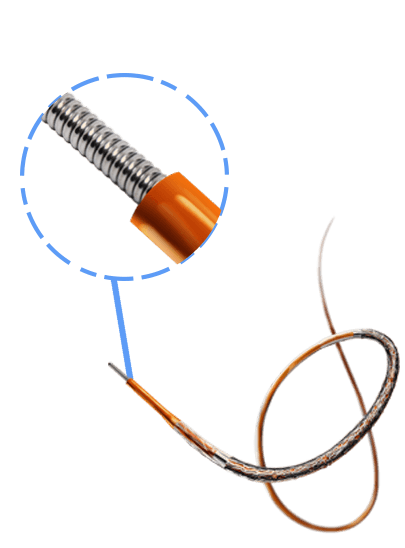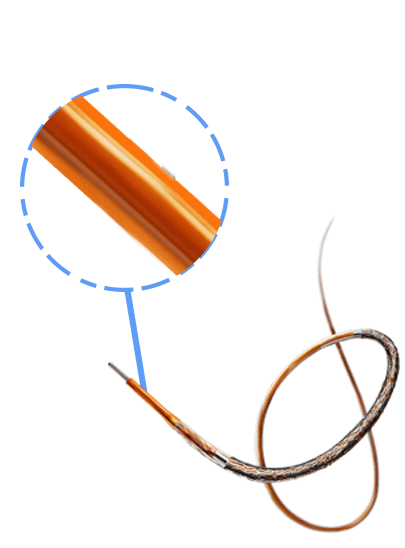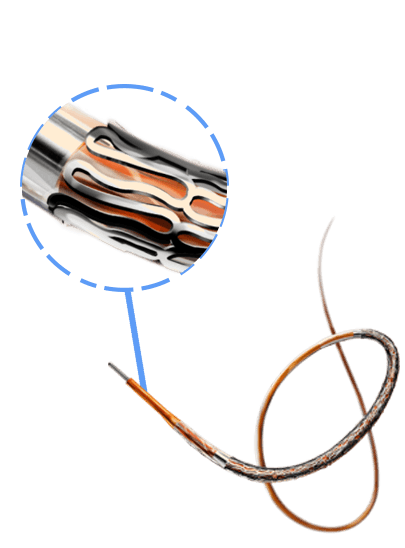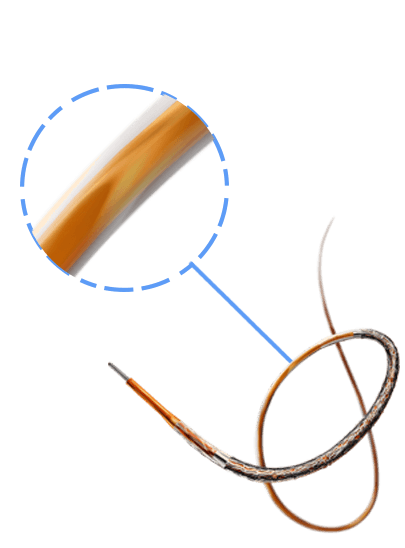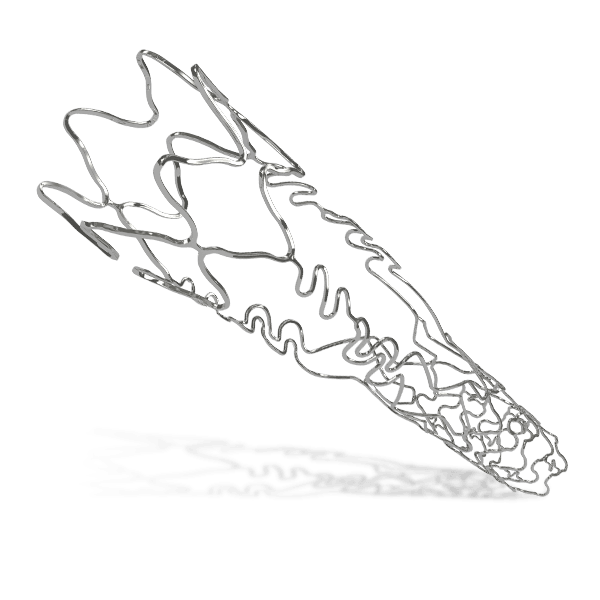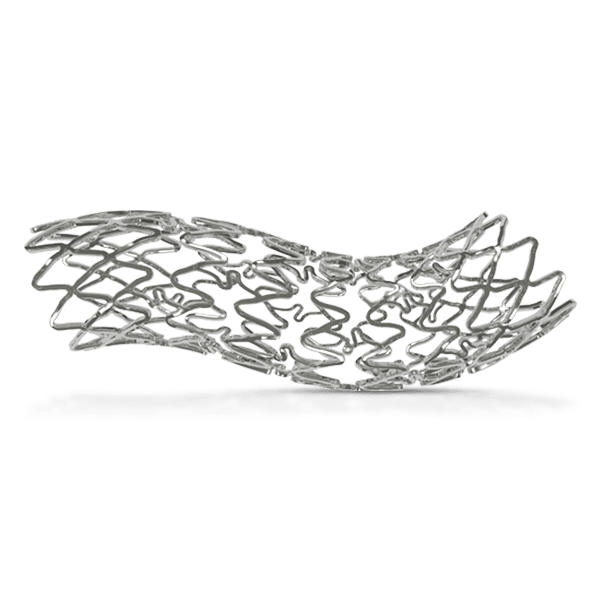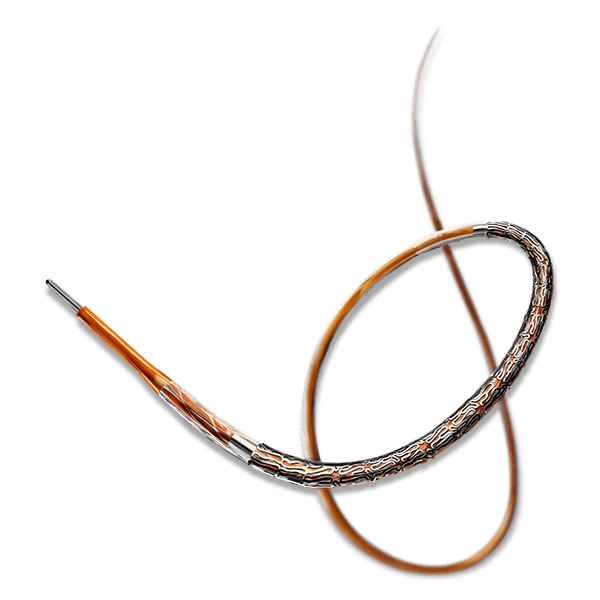
Breakthrough in DES Technology:
Designed to promote early vascular healing, this advanced stent features an ultra-low strut thickness of around 50 μm, minimizing vessel irritation while enhancing endothelialization. Its innovative structure incorporates a variable strut width and crown design, ensuring an optimal balance between flexibility and radial strength, with a robust performance of 1.1 bar. The hybrid cell design offers the best of both worlds—open cells in the middle enable seamless side-branch access, while closed cells at the ends provide superior scaffolding and conformability. Coated with the clinically established drug everolimus and a biodegradable polymer, this stent ensures proven long-term safety and efficacy, supporting superior patient outcomes.
*Bench test data on file at Meril Life Sciences
Key Features
Highlights
- >500 all comer patients studied in 3 clinical studies with follow-up upto 1 year. Ongoing robust clinical trial program
- 0% Stent Thrombosis at 6 months and 1 year in all comer patient population
- Low mace rate of < 2% at 1 year
Indication
Narrowing of a heart's blood vessel can result in its complete blockage and can progress gradually or can occur suddenly resulting in a heart attack, known as a myocardial infarction. Angioplasty is a procedure to widen the coronary artery and restore the blood flow.