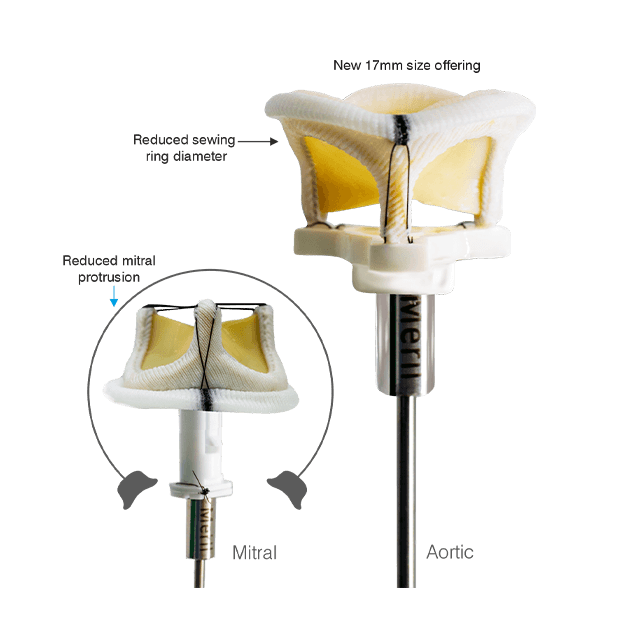
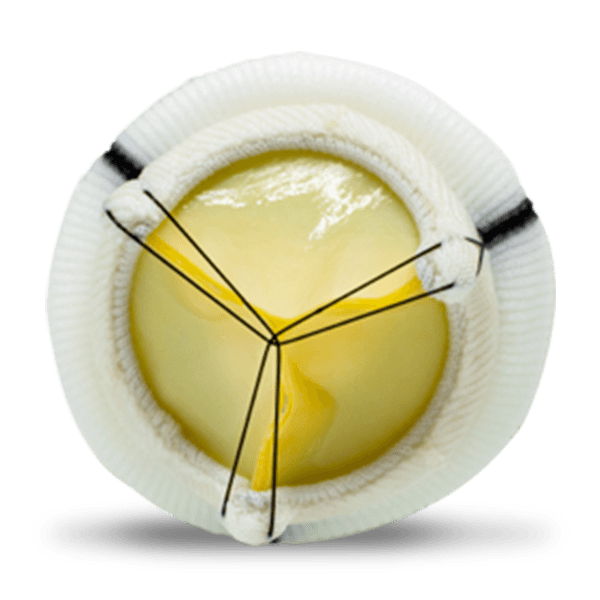
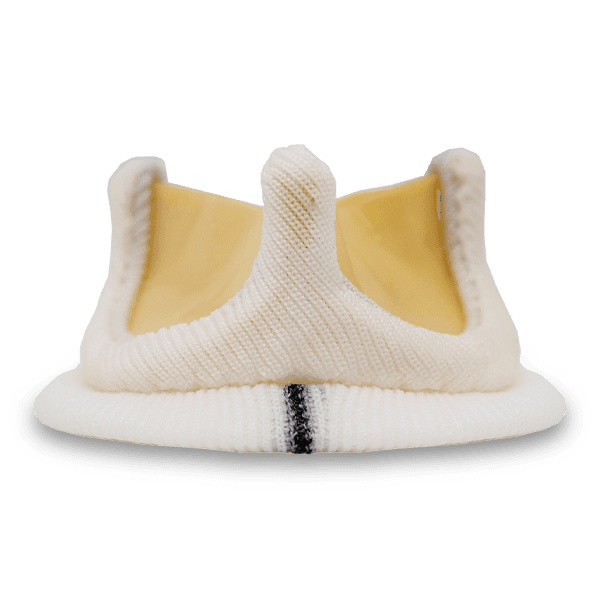
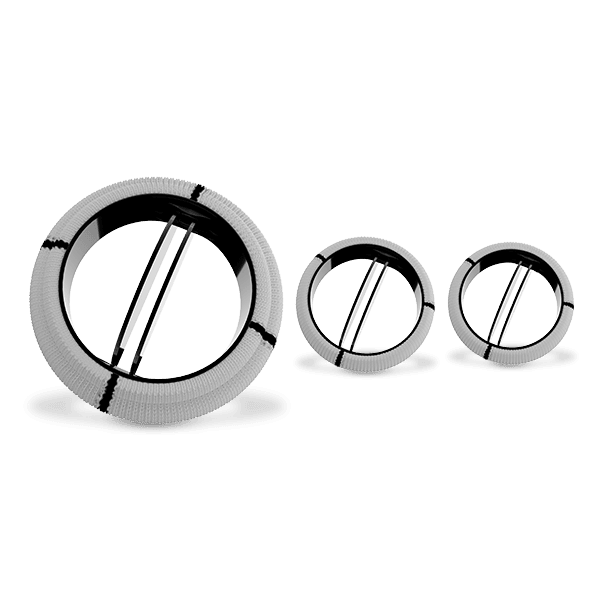Flomero™ Pericardial Bioprosthesis is a bovine pericardial, biomechanically engineered bioprosthesis featuring a triad frame design and triple composite leaflets mounted internally. Unique tissue fixation, state-of-the-art tissue thickness matching technology, advanced anti-calcification technology, and a mitral delivery system with a tenting facility translate into an efficacious product, enhancing Meril’s salient Tissue Heart Valve portfolio.
The triad frame comprises of a polymer ring (support ring), PET (polyethylene terephthalate) film structures (posts or commissures) and a frame made of Elgiloy alloy wireform. These three components of the frame are covered with polyester fabric and the frame is designed to be compliant at the orifice and commissures. A sewing ring made from polyester fabric is attached to the covered frame, featuring three contrasting markings to aid in the proper orientation for the implantation of the prosthesis.