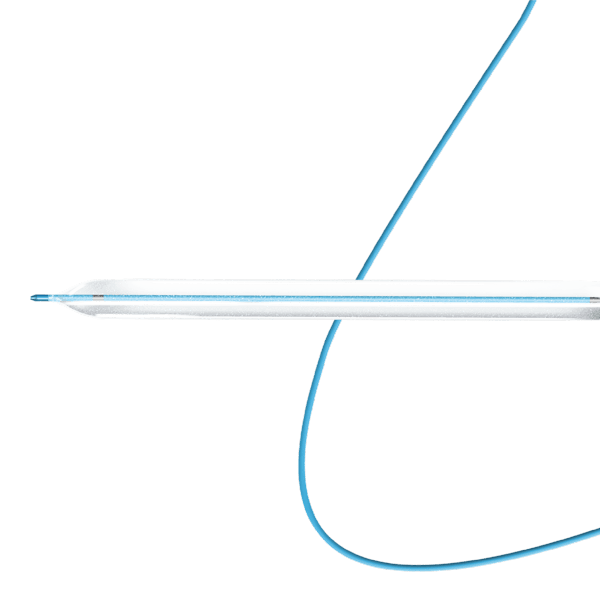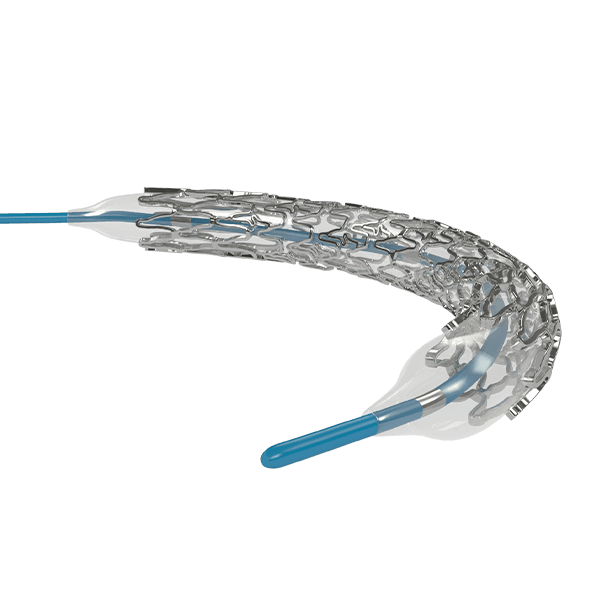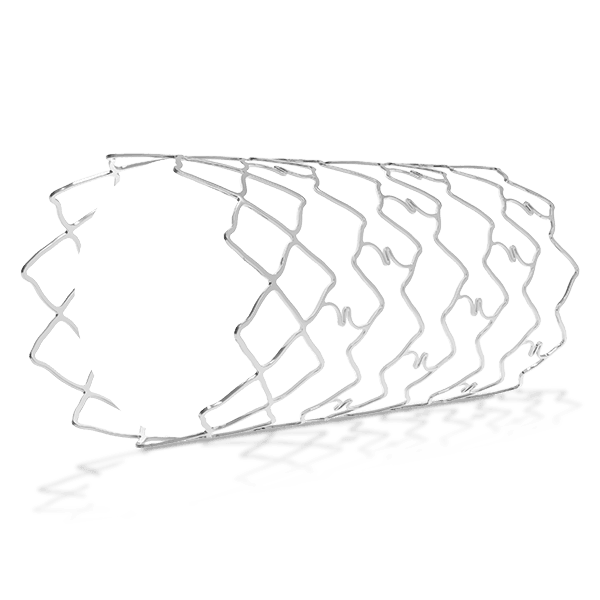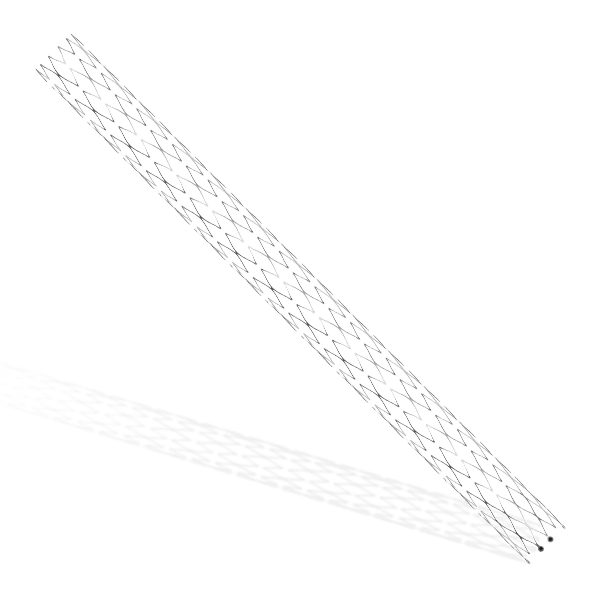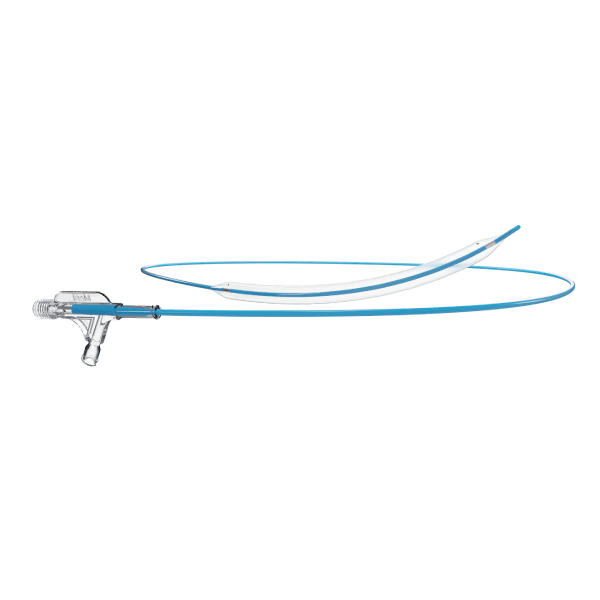
Traditionally, Plain Old Balloon Angioplasty (POBA) has been the primary treatment modality for patients with below-the-knee (BtK) arterial disease, especially in cases of critical limb ischemia (CLI). With advancements in vascular technology, the Credence BtK™ introduces a paradigm shift in BtK therapy.
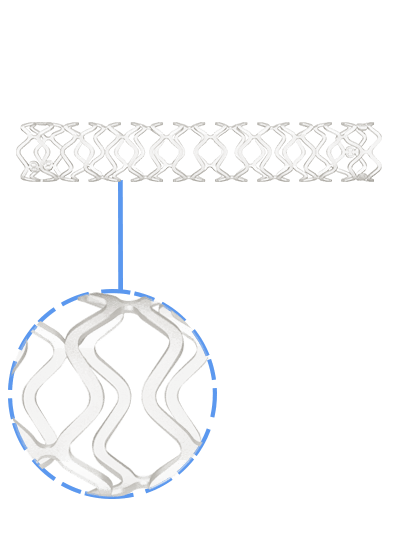
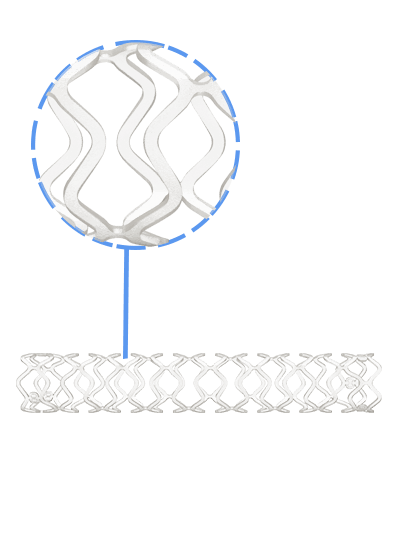
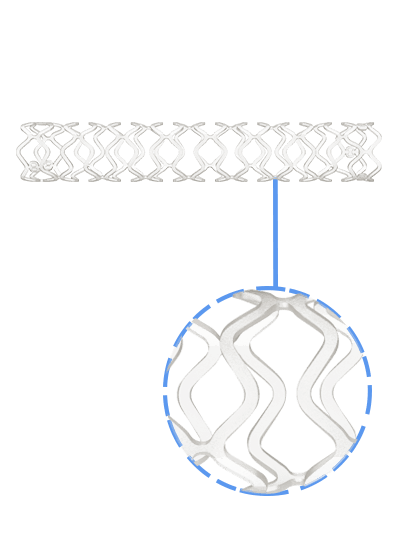
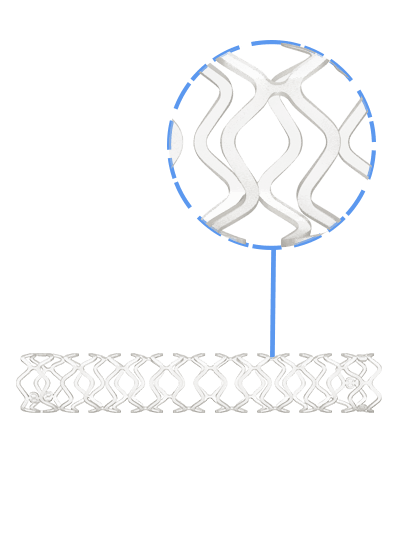
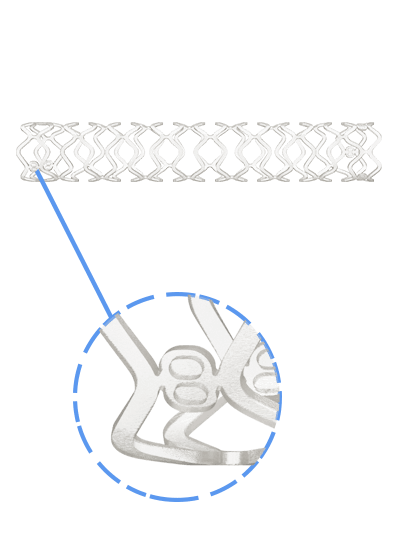
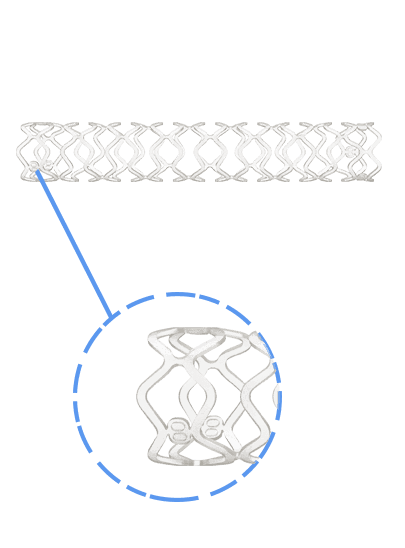
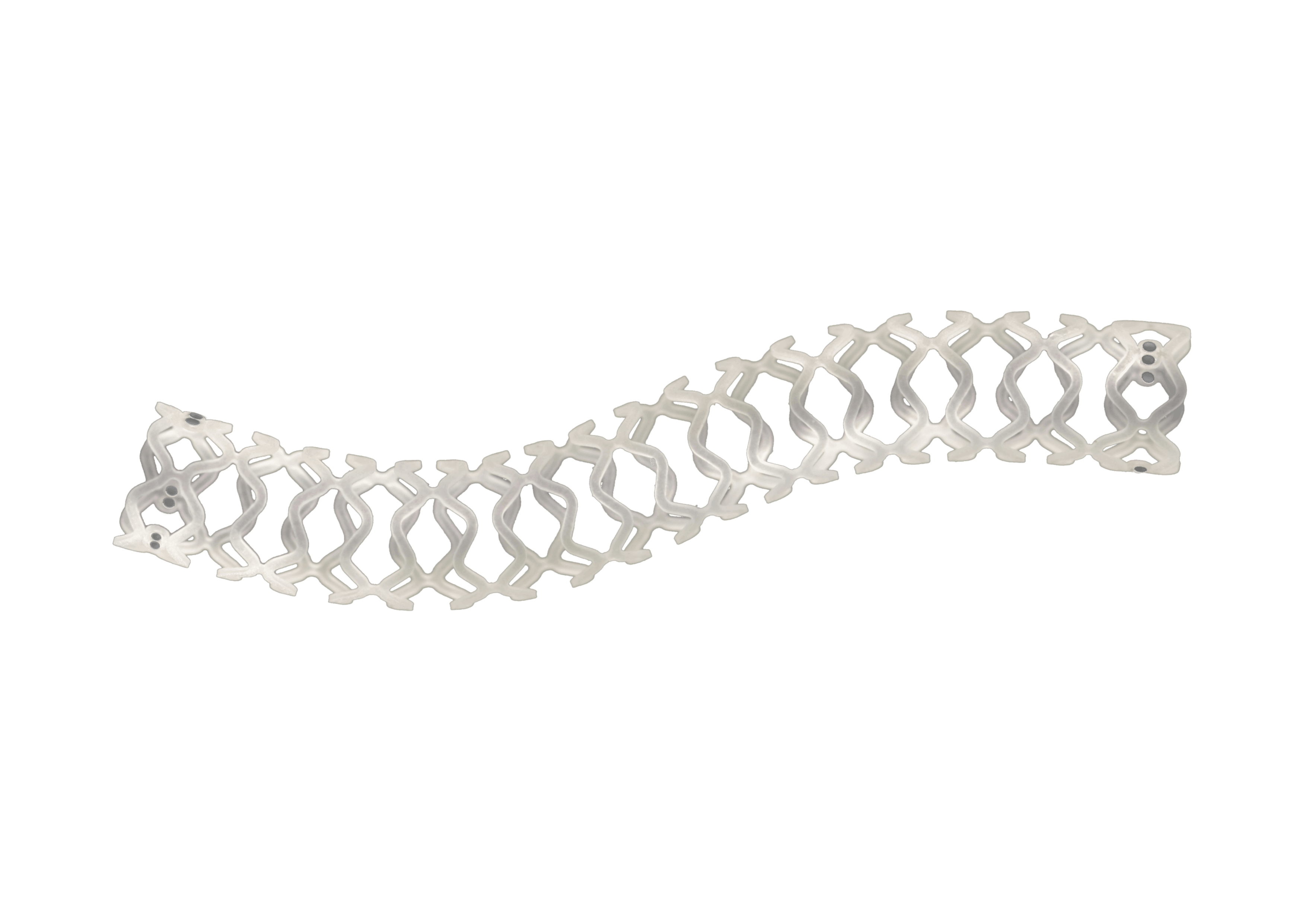
Credence BtK™ is a state-of-the-art bioresorbable scaffold engineered specifically for the treatment of critical limb ischemia and peripheral artery disease (PAD) in below-the-knee (BtK) arteries. Designed to provide temporary vessel support while eluting sirolimus, a proven antiproliferative drug, Credence BtK™ offers a dual benefit: immediate revascularization and long-term vessel restoration through natural healing.
Crafted with a fully bioresorbable polymer matrix, Credence BtK™ gradually dissolves over time, leaving behind no permanent implant. This eliminates chronic inflammation and reduces the risk of long-term complications associated with permanent metallic stents.