
HARNESSING THE POWER OF HYBRID
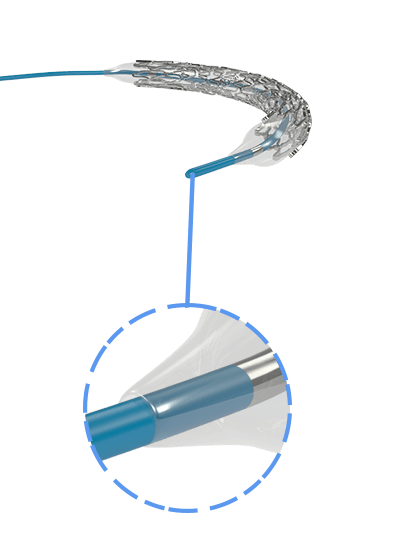
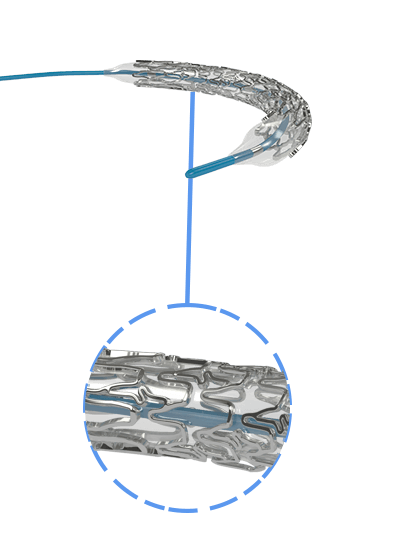
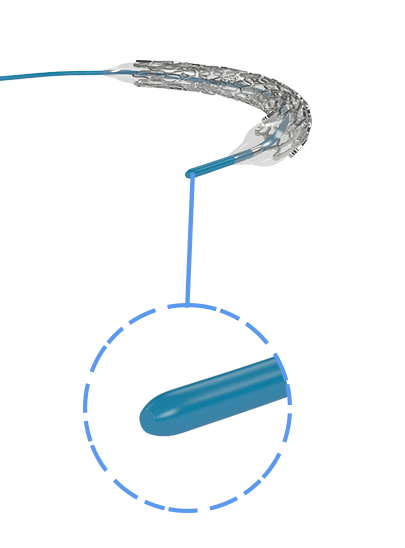
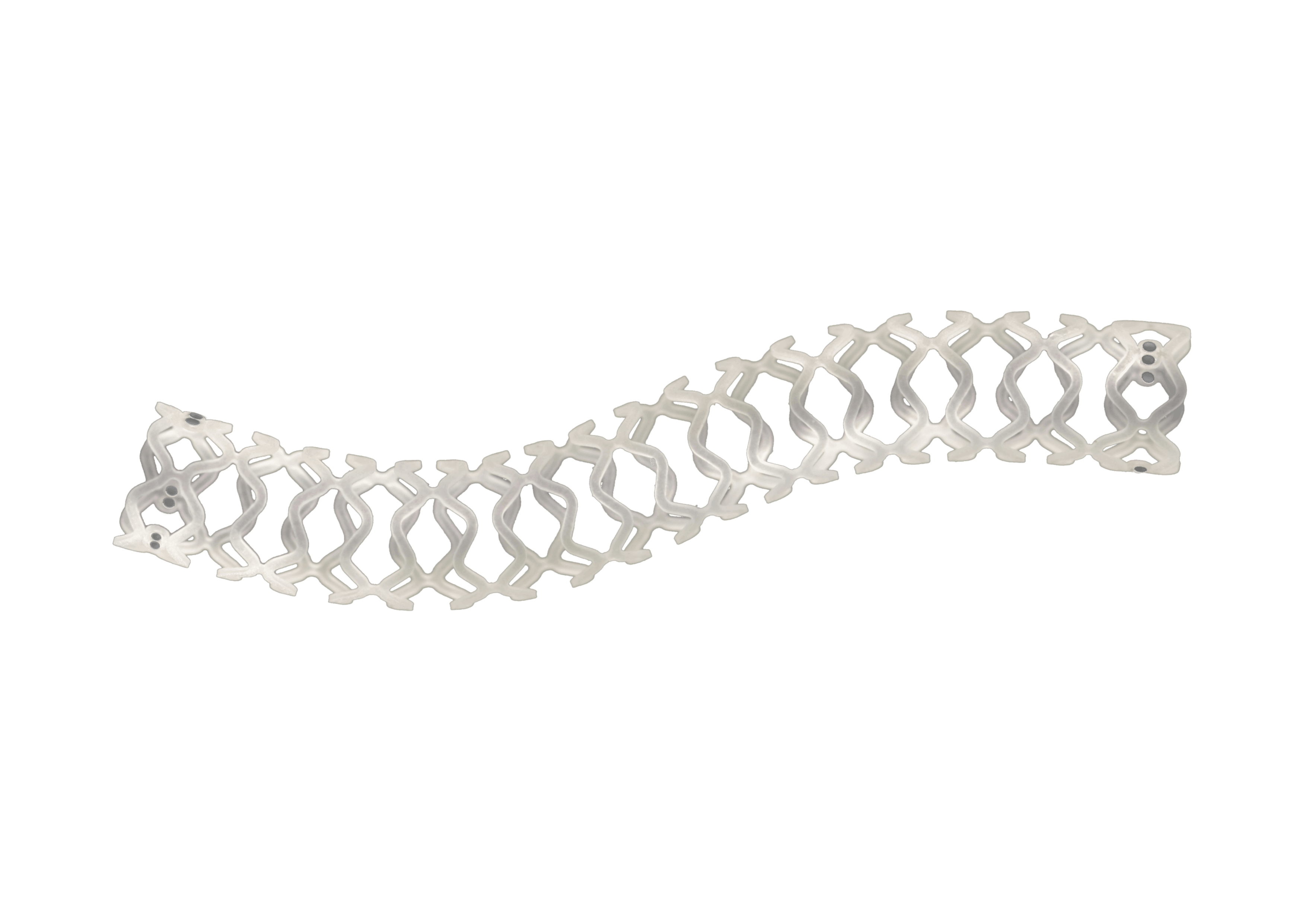
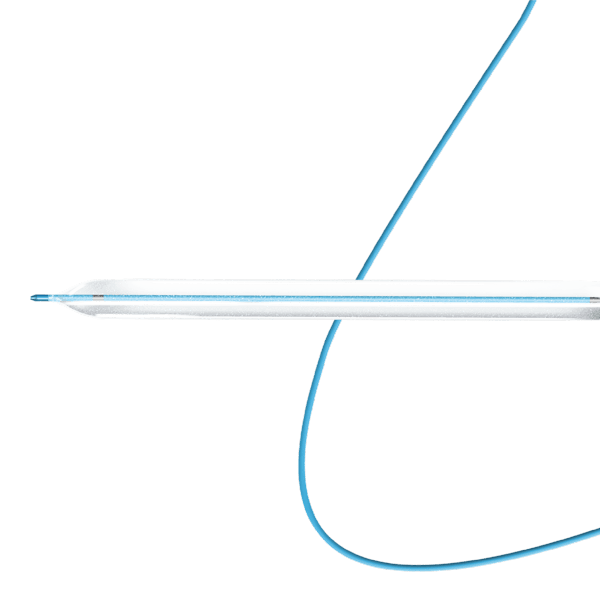
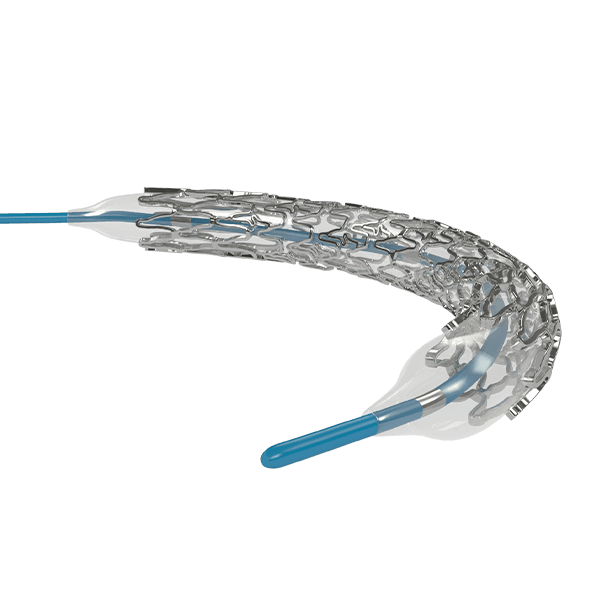
Myra™ BMS comprises of the following components:
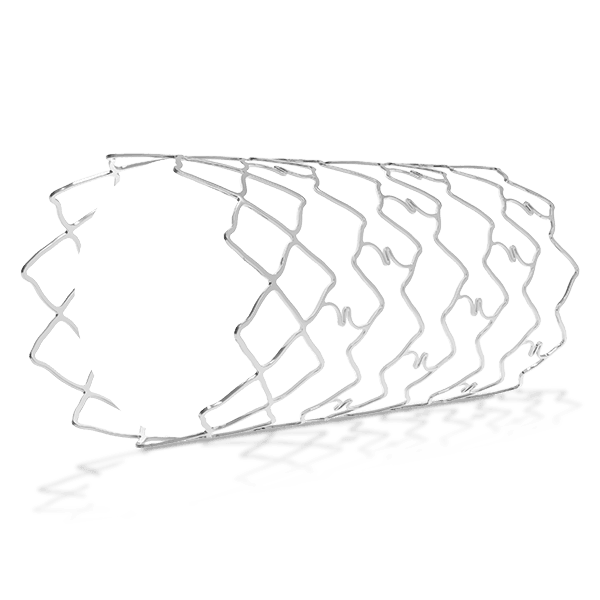
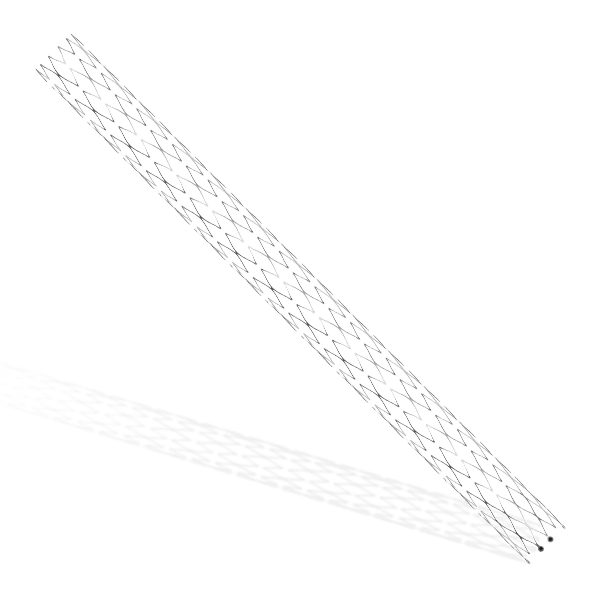
- A balloon-expandable L605 Cobalt Chromium Peripheral Stent
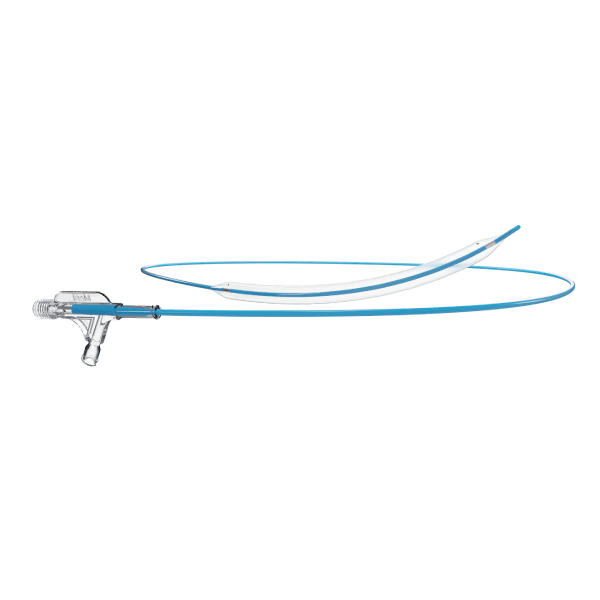
- An over-the-wire stent delivery PTA Balloon Dilatation Catheter
The stent is pre-mounted on Balloon Catheter & placed between two platinum-iridium radiopaque markers bands.
Key Features
Highlights
- Hybrid stent architecture offers strength & flexibility
- Short balloon overhang facilitates tracking and crossing of the stent delivery system
- Longitudinal flexibility to cross tortuous vessels and aortic bifurcation with contra-lateral approach
- Minimal foreshortening (<1.0%) ensures accuracy of placement
- 1.5–9 mm sheath is compatible with 6 Fr, and the 10 mm sheath is compatible with 7 Fr.
Indication
Intended for use in the treatment of atherosclerotic diseases of peripheral arteries below the aortic arch, with reference vessel diameter of 5.00 mm to 10.00 mm for Iliac / Sub-Clavian and for other protected peripheral arteries in patients eligible for Percutaneous Transluminal Angioplasty (PTA) and Stenting procedures.
FAQs
1. What is Myra BMS?
2. Where can Myra BMS be used?
3. What is special about its material?
4. Does Myra BMS provide accurate placement?
5. How is visibility ensured during the procedure?