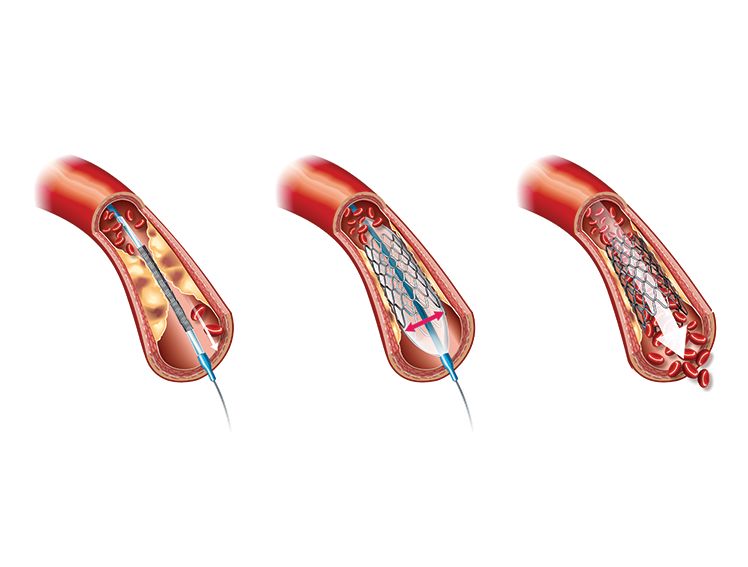
As of 2023, coronary artery disease (CAD) is the third leading single cause of mortality worldwide. It is associated with around 17.8 million deaths and trillions of dollars in healthcare costs annually1. Percutaneous coronary intervention (PCI) is a common modality for the treatment of CAD. Developed in 2003, this technique is used to remove plaques and mount a coronary stent at the affected location using catheters and guiding wires2.
The long-term effectiveness of implanting a coronary stent can vary based on the material of the stent2. Tracing the history of innovation in coronary stent design, one can see the progress from bare metal stents to those coated with anti-proliferative drugs, and ultimately, stents made of bioresorbable materials2. These innovations have allowed clinicians to overcome issues like excessive tissue growth around the stent, late stent thrombosis, and development of clots, while promoting healing and recovery3,4.
MeRes100, manufactured by Meril Life Sciences, takes bioresorbable stent (BRS) technology one step further. This is a next-generation stent made of poly-L-lactic acid that gets absorbed into the body swiftly and releases sirolimus to prevent adverse health effects associated with implantation. A salient feature of the stent is its 100-µm strut thickness, which makes it significantly thinner than its BRS predecessors, such as the Igaki-Tamai stent, manufactured by Igaki Medical Planning Company in Kyoto, which is 170 µm thick and the BVS everolimus-eluting stent manufactured by Abbott Vascular in Santa Clara, which is 156 µm thick5. Lower strut thickness is known to enable better post-stent outcomes and healing6.
MeRes100 also provides optimized mechanical support to ensure faster healing and minimal side effects. In the initial stage after angioplasty, MeRes100 has high radial strength. Here, it acts as a typical drug-eluting stent to speed up arterial healing and restore blood flow. During the next phase, which lasts 3 to 6 months after surgery, the stent gradually loses radial strength, ultimately undergoing complete degradation during the final stage, when the artery walls no longer require mechanical reinforcement. The scaffold gets completely resorbed, with the release of carbon dioxide and water, over a period of 24 to 36 months post angioplasty.
The first and second trials of MeRes100, published in 2017 and 2019, respectively, found that it was associated with a low lumen loss at 6 months after implantation7,8. The second trial additionally revealed that lumen loss did not change significantly from 6 months to 2 years after angioplasty. MeRes100 provides nearly complete strut coverage, sustained medium flow area, and very low percentage volume obstruction after implantation. The trials also suggest that it may lower the risk of scaffold thrombosis compared to other first-generation resorbable stents.
Next-generation stents, such as MeRes100, exhibit markedly enhanced performance in terms of drug delivery efficiency and degradation speed compared to their first-generation BRS counterparts. Utilizing these stents can substantially diminish the long-term adverse events associated with the permanent metallic implants. Have we reached the pinnacle of stent technology for PCI?
References
Brown, J. C. (2023, January 23). Risk factors for coronary artery disease. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK554410/
Ho, M., Chen, C., Wang, C., Chang, S., Hsieh, M., Lee, C., … & Hsieh, I. (2016). The development of coronary artery stents: From Bare-Metal to Bio-Resorbable Types. Metals, 6(7), 168. https://doi.org/10.3390/met6070168
Nicolas, J., Pivato, C. A., Chiarito, M., Beerkens, F., Cao, D., & Mehran, R. (2022). Evolution of drug-eluting coronary stents: a back-and-forth journey from the bench to bedside. Cardiovascular Research, 119(3), 631–646. https://doi.org/10.1093/cvr/cvac105
Zong, J., He, Q., Liu, Y., Qiu, M., Wu, J., & Hu, B. (2022). Advances in the development of biodegradable coronary stents: A translational perspective. Materials Today Bio, 16, 100368. https://doi.org/10.1016/j.mtbio.2022.100368
Ormiston, J. A., & Serruys, P. W. (2009). Bioabsorbable coronary stents. Circulation: Cardiovascular Interventions, 2(3), 255–260. . https://doi.org/10.1161/circinterventions.109.859173
Iglesias, J. F., Degrauwe, S., Cimci, M., Chatelain, Q., Roffi, M., Windecker, S., & Pilgrim, T. (2021). Differential effects of newer-generation ultrathin-strut versus thicker-strut drug-eluting stents in chronic and acute coronary syndromes. Cardiovascular Interventions, 14(22), 2461-2473. . https://doi.org/10.1016/j.jcin.2021.09.028
Seth, A., Onuma, Y., Costa, R., Chandra, P., Bahl, V. K., Manjunath, … & Serruys, P. W. (2017). First-in-human evaluation of a novel poly-L-lactide based sirolimus-eluting bioresorbable vascular scaffold for the treatment of de novo native coronary artery lesions: MeRes-1 trial. Eurointervention, 13(4), 415–423. https://doi.org/10.4244/eij-d-17-00306
Seth, A., Onuma, Y., Chandra, P., Bahl, V. K., Manjunath, C. N., Mahajan, A. U., … & Serruys, P. W. (2019). Three-year clinical and two-year multimodality imaging outcomes of a thin-strut sirolimus-eluting bioresorbable vascular scaffold: MeRes-1 trial. Eurointervention, 15(7), 607–614. https://doi.org/10.4244/eij-d-19-00324
SHARE NOW